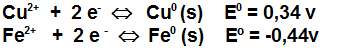
(the potential here is the reducing one)
In a galvanic cell, the cathode is the metallic copper submerged into a solution of Cu 2+ of 1 mole / littre, and the anode is the metallic iron submerged into a solution of Fe 2+ of 1 mole / littre. The cell potential is...
(a) 0,10 V
(b) –0,10V
(c) 0,78 V
(d) –0,39 V
(e) –0,78 V
The answer is (C), but I want to confirm if my reasoning is correct.
The copper wants to reduce, and the iron wants to oxidize, so if the copper is the cathode and the iron is the anode, then the current is natural, and thus it is possible.
If the copper were the anode and the iron were the cathode, then the sign would be negative (option E = -0.78 V).
Am I correct?