Borek..
(1) You copied and pasted a 1996 "unauthenticated" something and a 1993 publication
by Blackwell publications that calls itself a "guide" and refers to the IUPAC rules and
states that E/Z has largely replaced cis / trans. I'm not arguing with that.
(2) The original question was "how can someone identify cis and trans". right? It wasn't
"has cis / trans been replaced with E/Z" was it?
(3) IUPAC does in fact discourage the use of "cis" and "trans" in favor of E-Z in their preferred names
see "Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names, 2013" by
Favre and Powel (that is the IUPAC BLUE BOOK published by IUPAC with their rules in it. The horses
mouth). Chapter P-9 has the relevant rules. But again, the question wasn't which is the preferred
system was it?
continuing with the actual IUPAC blue book.
here's an image from P-92.1.1(e)
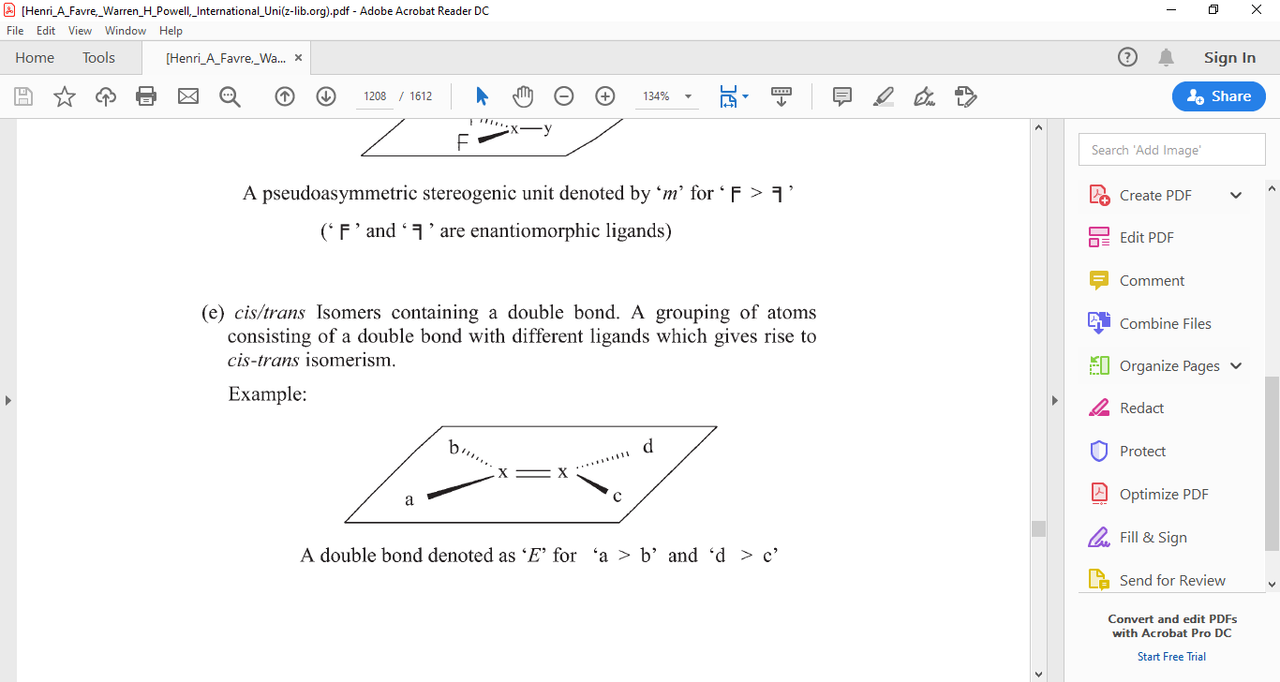
notice the 4 ligands
P-92.1.2 states that CIP rules are used to prioritize a,b,c,d
P-92.1.3 defines the rules to determine order of precedence.. each to be applied exhaustively before
the next is applied. in brief they are:
(1) higher atomic number has priority
(2) higher atomic mass has priority
(3) when considering double bonds with tetraligand atoms "seqcis" = "Z" > "seqtrans"="E"
(4) refers to chiral centers
(5) states that R > S
P-92.4 explains "rule (3)" in detail and states again that "seqcis" = "Z" and "seqtrans" = "E"
and states that Z and E are PIN (Preferred IUPAC Nomenclature) and states that
Z and E are derived from the German words "Zusammen" and "Entgagen" (same and opposite)
here's an image you might find interesting from P-92.6
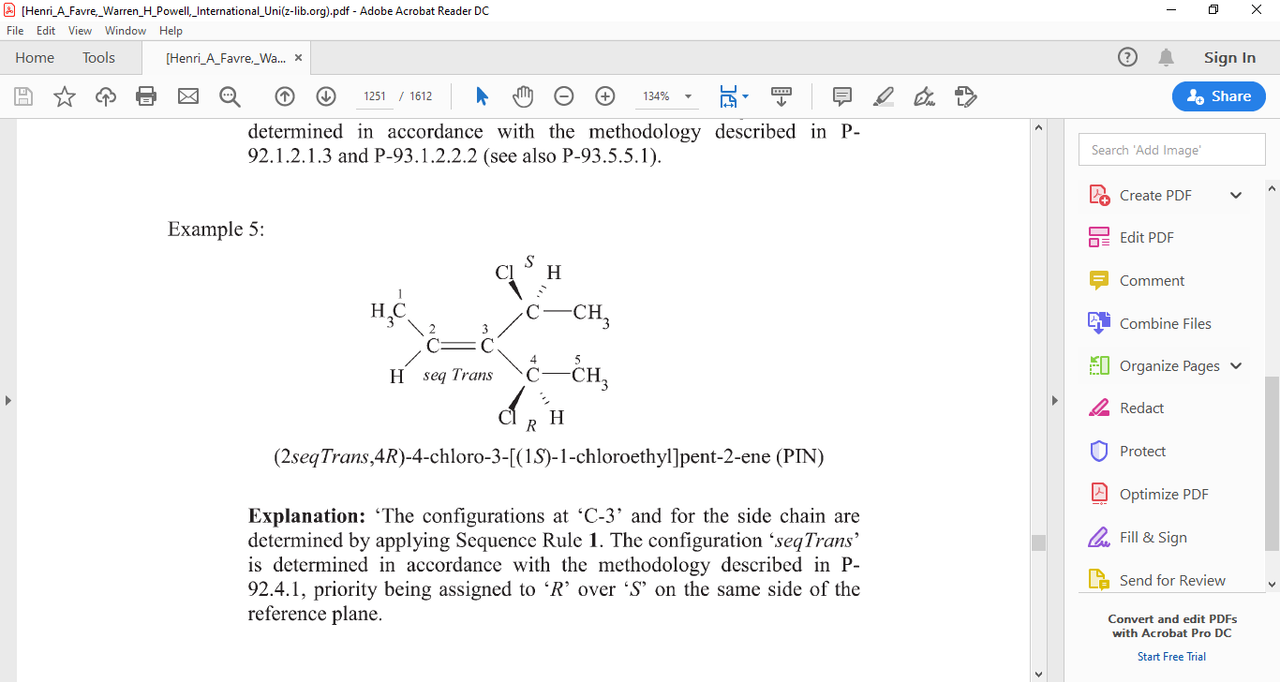
where IUPAC determined by rule (1) that CH3 > H and by rule (5) R>S and called this thing "TRANS"
not "E" in the PIN
The rest of the chapter is focused giving examples of those 5 sequencing (or priority) rules.
(4) IUPAC isn't always consistent. They seem to equate "cis/trans" with "E/Z" and USUALLY declare
E/Z to be the PIN. But not always.
(5) You can see by my examples, there is a world packed full of chemical manufacturers, suppliers,
marketing folks, researchers, chemical users, government agencies, etc that use "cis/trans"
nomenclature even when there are 3 or more ligands (non-H atoms) attached to the double
bonded C's. Argue if you like... but all those MILLIONS of people do indeed use cis/trans.
And they determine which is which exactly as I described.
back to the original question.. "how do you determine cis vs trans". My answer is accurate.