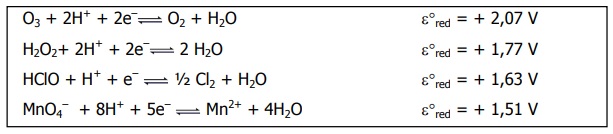
Hello
I was given this exercise and some options, so I have to mark the correct one.
a) an aqueous solution of hypochlorite can oxidize ions Mn
2+b) an aqueous solution of H
2O
2 is a strong reducing agent
c) ozone leans strongly to giving out electrons in an aqueous solution
d) the addition of H
2O
2 to an aqueous solution--having dissolved oxygen--promotes the formation of gas ozone
e) permanganate, among other substances in the image, is the most powerful oxidizing agent
I thought that all I had to do was look at the image and see the respective voltages, right? So I just marked (a) right away, because 1.63 V > 1.51 V, and I got it right.
But the thing is, after reading (d), I'm not so sure anymore if that's all I had to do. Perhaps I just found the correct answer by serendipity, and I actually had to play a bit with the reactions?
Can you guys confirm it to me?
Thank you very much