I'm a little confused about the discussion here. This problem is clearly
(1) an example that volume is not conserved but mass is
(2) has fake densities (or at least typo-ed densities)
just to be clear
volume 1M H2SO4 + volume H2O ≠ volume mixture
mass 1M H2SO4 + mass H2O = mass mixture
moles H2SO4 in 1M H2SO4 = moles H2SO4 in dilute solution.. the moles don't change
steps to solve this
(1) convert volume 1M H2SO4 to mass 1M H2SO4 using density H2SO4
(2) convert volume H2O to mass H2O using density of H2O (not given)
(3) add the masses to get mass solution
(4) convert mass solution to volume solution using density of dilute solution
(5) at this point you can either do this
from a mole balance M1V1 = M2V2
or
convert 100.mL 1M H2SO4 to moles H2SO4, then divide by volume solution
*****
I'll demonstrate the math, you all can correct the calcs with the correct densities if you like
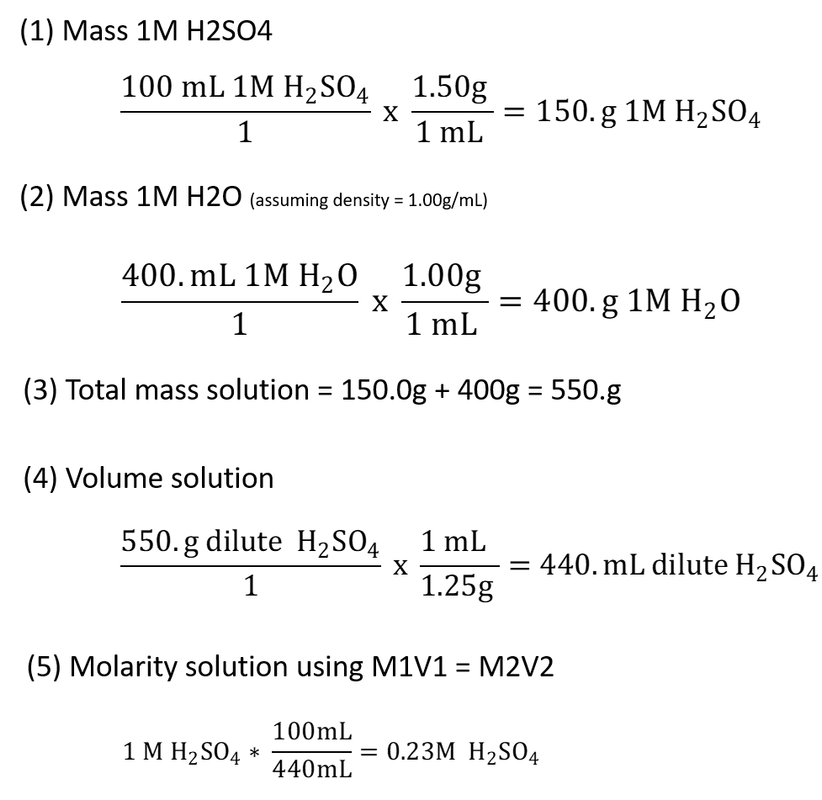
this is NOT correct
M2 = 1M * (100mL / 500mL) = 0.20mL
One of you all can update the densities and recalc this if you wish.