I am studying chemistry at uni and I had a question which asked me write the skeletal formula of all 3 isomers of the above compound. I worked them out myself, then I went and researched them on the net to confirm I was correct. But I came up with a 4th isomer myself and can't find the flaw in my logic. I cannot find this 4th isomer anywhere on the Internet so I am confident I am wrong in this, but I just want someone to explain to me where my logic is flawed. Here are the facts what I was presented with:
chemical formula: C
6H
13Br
first isomer - 1-Bromo-3-methylpentane
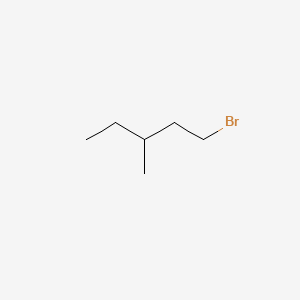
This has the Br element hanging off the end of the chain
second isomer - 2-Bromo-3-methylpentane
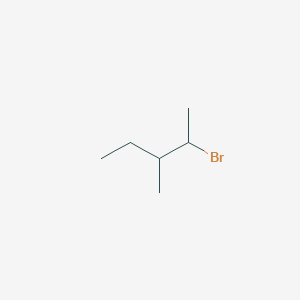
This has the Br element hanging off the 2n or 4th carbon
third isomer - 3-Bromo-3-methylpentane
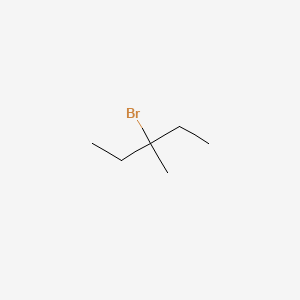
This has the Br element coming off the 3rd carbon in the opposite direction of double carbon branched chain.
I came up with a 4th, it is the same structure as the 3rd isomer but instead of the Br being where it is there, I had it on the opposite side - at the very end of the C-C branch.
Why is this not a valid isomer?