Hi! I'm doing a level chemistry and I'm currently revising the transition metals topic. I understand ligand substitution of the equilibrium equations but I just can't understand why ammonia substitutes water in the way it does...
[Cu(H
2O)
6]
2+ + 4NH
3 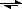
[Cu(NH
3)
4(H
2O)
2]
2+ + 4H
2O
So, if the above is what occurs when you add excess ammonia
the position of equilibrium of:
[Cu(H
2O)
6]
2+ + 2NH
3 
[Cu(H
2O)
4(OH)
2] + 2NH
4+shifts to the left (correct me if I'm wrong)
So, I get what's happening ;but why?
Why does the ammonia substitute 4 of the water ligands?
In my head, it's not due to the chelate effect as it isn't entropically favourable, as the number of mols is equal on both sides of the equation.
Additionally, why partial substitution and not complete?
Lastly, why does it substitute to become [Cu(NH
3)
4(H
2O)
2]
2+ and not Cu(NH
3)
4(OH)
2 ?
Sorry, I know I've asked a lot of questions there and that the answers are complicated and I definitely don't need to know the answers for my exam, but I'm just wondering as my teachers won't go into detail about it.
Thank you ! (also Merry Christmas!)
