Getting back to the original question,
The best way to predict the pKa of molecules is to memorize a list of some basic ones, like acetic acid, the mineral acids, protonated amines, alcohols, etc. Then, when you see a complex molecule like this, you can say "oh, this is about the same as acetic acid, so the pKa is around 5."
You are correct in saying that the potassium form is less acidic. It should also be more water soluble, due to the dissociation of the cation and anion. The acid form would not dissociate much in pure water because it needs a base to deprotonate it. In pure water, the equilibrium would look like:
R-COOH + H
2O
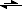
R-COO
- + H
3O
+In this case, the hydronium ion is a much stronger acid than the carboxylic acid, so the equilibrium will be shifted to the left.
Also, sometimes big carboxylic acids like this can dimerize and be completely insoluble in water, as in the case of benzoic acid.