NH
3 + B
-  -
-NH
2 + BH
H≡H + B
- 
H≡
- + BH
I do not have a clear understanding of this stuff and I tend to get confused. I do understand that in order to deprotonate an acidic proton, the pka of the conjugate acid of the base used must be greater than the pka of the acidic proton for the equilibrium shift to the right.
I think I kind of get what you are trying to make me learn here. NH3 is not a strong enough base to deprotonate the ethyne as the conjugate acid of ammonia,
+NH
4 has a pka of ~4.5 which is actually more acidic than the ethyne proton. Thus why we need the sodium amide. and ammonia is just there as a solvent or like water for sodium hydroxide?
So should I be writing it as
NH
3 +
-NH2
 -
-NH
2 +
+NH
3?
an endless supply of
-NH2?
I just got confused about something else. FMOC deprotection, that carbon in the FMOC which gets deprotonated has a pka of 22.6, how is it that piperidine which has a pka of 11ish is able to deprotonate it? Would not the equilibrium be more to the left in such a case in the following equation?
R
2N-FMOC + piperidine
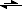
R
2N-
-FMOC(-H) +
+Piperidine-(+H)
Is it because the acetate is a better nucleophile and picks up the piperidine proton before
-FMOC(-H) has a chance?
Bleh =(
Nescafe.