As a matter of fact, I was looking at ionization energies of the 2nd row elements and trying to figure out why Be and N have higher ionization energy than B and O respectively.
The explanation for the Be and B difference is in the electron configurations: Be 1s2 2s2, B 1s2 2s2 2p1
The 2p orbital has a bit higher energy than 2s orbital, and less energy is required to take away that electron and ionize boron. Also, my textbook (and a quick google search) tells me that 2s2 is somehow stable because of "quantum mechanical" reasons. No specifics, just that.
As for N and O we have these: N 1s2 2s2 2p3 and O 1s2 2s2 2p4
with N
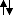



and O
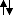
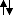


The textbook is a bit useless here, but I found somewhere that that extra electron that's paired up in the p orbital is repulsed by others and the raise in the nucleus charge can't compensate that somehow.
Now, you say that the closer the electron to the nucleus, the harder it is to remove it - that makes perfect sense; no other electrons to repulse it, and the closer to the core, the stronger the attraction.
But what does it mean when they say that 1s1 electron has the lowest energy state? You need more energy to remove it than an electron in a f orbital and that same electron is in a higher energy state?
Higher energy state = more energy to remove it? Do you see what's so confusing? more energy=less energy - HOW?
Can someone help disentangle me from this semantic web that I have fallen victim to? Please?
