I have no idea... (goes off to Google it)
Found this
http://jcp.aip.org/resource/1/jcpsa6/v44/i5/p1748_s1?isAuthorized=noApparently, you can't use electron diffraction to figure out the structure ... strange. I thought you use neutron diffraction to see small molecules like Hydrogen. I guess I have a lot more to learn...

They also say "The PtF
6- ion is a regular octahedron with a Pt☒F bond length of 1.82±0.03 Å."
So it's like
http://upload.wikimedia.org/wikipedia/commons/0/07/Octahedron.svg, with the Pt atom inside it.
The O
2+ bond to PtF
6- is ionic. Same thing with Xe
+. That F really can't get enough of electrons. What a selfish prick of an element...
As EpicWinston said, F is extremly electronegative. So if it can take an electron from O
2, it can take it from Xe too.
Also I forgot to say that in Xe
5s 5p 5d
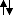
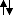
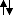


and
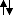
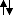




The first one makes two covalent bonds and the second one four.
In Xe
+ it's probably the first case where you have two unpaired electrons and one electron is being pulled away more than usual by fluorine, making a cation. The other one is covalent? Don't have a clue really. Maybe it just stays in the Xe p-orbital, making the whole thing paramagnetic, or not. I'll see what I can find.