What about this one though?
Do I do the same thing with the Birch Reduction?

Like this?
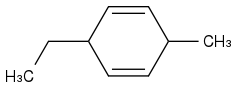
Because this is what I had in my notes...
In the first electron transfer, the carbon to which the sodium electron adds creates a carbanion and the carbon "para" to the carbanion is a carbon radical which becomes a carbanion upon electron transfer from the second sodium atom. Since the carbanion is negatively charged, it would not be low in energy if it formed on a carbon bonded to an alkyl group (an electron pump). This results in the alkyl group being bonded to a double bond carbon (sp2 carbon) and not to an sp3 carbon.