It is not a diol, so maybe the periodate oxidized the diol to compound A,
Yes, as well as acting as the stoichiometric oxidant in the osmium-catalysed dihydroxylation, periodate will oxidatively cleave the diol (periodate cleavage).
and as M is an olefin it should be methyl-cyclobutane, but how could it be obtained from compound L?
I am confused by this. methylcyclobutane is not an alkene....
Assuming that your structure for A is correct (which I think it is), I would guess that M is:
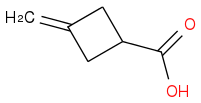
Also, from the first part, I think N might just be HBr.