I don't really understand how you get from the reactants to the products.
I know that.
You are almost there, what you have suggested so far is correct, you just need to finish it.
You know you have this intermediate so far:
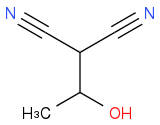
You know CH
2(CN)
2 (malononitrile) is one of the final products. Draw a circle around the part of the intermediate that corresponds to malononitrile. Which bond needs to break to make malononitrile?