I was reading a textbook and got confused with the reaction of oxacyclopropanes.
For example:
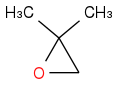
If we treat the above compound in basic condition with Grignard the Grignard reagend would attack the less sterically hindered C atom which is the one right to the oxygen in the ring.
Then, if we treat the same compound with Grignard in acidic conditions, the oxygen gets protonated and the Grignard will attack the sterically hindered C atom because the transition state is more stable.
Now to the thing that confuses me. If we treat a compound like:
![CC1(C)[Br+]C1](https://www.chemicalforums.com/SMILES/8644f8bd7db9ab3e1884.png)
With OH
- regardless of acid/base conditions it should attack the sterically hindered C atom because it has a bigger partial positive charge than the other C atom. This seems to be in contradiction with the previous examples. Why wouldn't Grignard attack that C atom because of the positive charge in the previous example? I need some clarification here.