While in typical organic chemistry reactions, activation may act primarily by altering the kinetics of a particular reaction, in biochemistry, activation steps act primarily to alter the thermodynamics of a reaction.
For example, in the case of glycogen synthesis, the enzyme glycogen phosphorylase catalyzes the joining of an unactivated glucose-1-phosphate to glycogen, using inorganic phosphate as the leaving group:
glucose-1-phosphate + glycogen
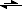
glycogen+1 + Pi
Of course, because the concentration of inorganic phosphate in the cell is so high, what actually occurs when glycogen phosphorylase gets activated is the breakdown of glycogen (i.e. the reverse of the reaction written above). However, if one were to put put glycogen with the enzyme in the presence of high concentrations of glucose-1-phosphate and low concentrations of inorganic phosphate, the enzyme would likely have no trouble catalyzing the synthesis of glycogen at a reasonable rate.
Thus in this case. the kinetics of the reaction is not the problem, and activation of glucose-1-phosphate with UDP is required in order for glycogen synthesis to be thermodynamically favored.