
For the bottom reaction, wouldn't the product be this (when the C=C double bond on the far right breaks as 2 electrons from it get pushed onto the top carbon, which then attacks the H-H and the H nucleophile attacks the electrophilic carbon):
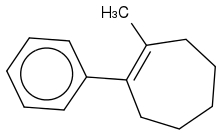
For the IUPAC I wrote, cis- 1-methyl-2-phenylcyclohept-1-ene (ene for the double bond at 1!) But that's wrong

The answer is supposed to be
cis-1-methyl-2-phenylcycloheptaneI don't understand

1) Do BOTH C=C double bonds in cycloheptane get reduced to CH-CH because it's EXCESS H2/Pd? Also did I describe the Excess H2/Pd mechanism correctly?
2) Does the correct answer look like this?
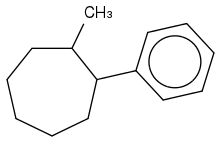
3) Why wouldn't I reduce all the double bonds in the benzene? Is it because H2/Pd catalyst in general can't because it's not a strong enough reagent and it's only used for alkenes?
4) How exactly do you name a structure like this? I know that cis means 2 things are on the SAME side of a bond (hence on the far right of the cycloheptane structure, the H's are sticking OUT on the same side of the C-C bond because there's a lot of space/elbow room, and on the top left of the cyclheptane structure, the H's would be sticking INTO the page while being on the same side of the C-C bond because it's crowded). Is my reasoning correct?