Take but-1-ene and treat it with HBr in the presence of peroxides followed by addition of sodium cyanide. To this product add aqueous acid and heat it up.
(a) Draw the major organic product that results from the 3-step synthesis above.
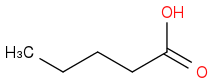
(CORRECT)
Take the MOP you drew for part a and treat it with phosphorus tribromide. To that product add molecular bromine then follow that up with an aqueous workup.
(b) Draw the major organic product that results from the second 3-step synthesis.
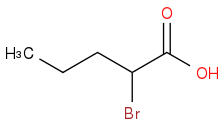
I understand the answer to part A, but HOW is the answer to part B that?
OH is essentially supposed to be replaced by Bromine to give you an acyl bromide, no? Instead, it adds to the alpha carbon.
I'm so confused.. Why is that???