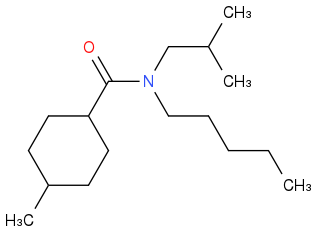
I'm starting with Organic Chemistry and the nomenclature. I just invented a structure, but the freeware version of ChemSketch doesn't tell me the name of molecules with more than 50 atoms, so I'm looking for a confirm.
It is a tertiary amine. It is bound to a pentane and to a 2-methylpropane.
So it is:
N-2-methylpropyl-N-pentyl-.
The first atom of the cycle is the one bound to the -COOH. So we have a 4-methylcyclohexane.
The name should be:
N-2-methylpropyl-N-pentyl-4-methylcyclohexanecarboxiamide.
Am I right?
I'm just interested into a hypothetic name.
PS. I'm italian, I don't really know if the order is different in some parts (for example butanoic anhydride is "anidride butanoica" in italian)