Making predictions is great. But data doesn't lie.* Let's look at some real combustion data, see what we see.
Combustion data is freely available for many organic compounds at the NIST chemistry webbook.
http://webbook.nist.gov/chemistry/Let's consider your first question: does the number of carbons in a primary alcohol affect the combustion enthalpy. You predicted that it would increase. Actually, it decreases. (I imagine you meant to say that the absolute magnitude would increase. The combustion enthalpy is negative since the reaction is exothermic.) Here is the data (averaged, because multiple experimental values are provided, values are in kJ/mol):
| Alcohol | | # of carbons | | ΔHc | | ΔHc per carbon |
| Methanol | | 1 | | -721 | | -721 |
| Ethanol | | 2 | | -1368 | | -684 |
| Propanol | | 3 | | -2022 | | -674 |
| Butanol | | 4 | | -2670 | | -668 |
| Pentanol | | 5 | | -3328 | | -666 |
| Hexanol | | 6 | | -3981 | | -663 |
| Octanol | | 8 | | -5290 | | -661 |
| Decanol | | 10 | | -6601 | | -660 |
| Dodecanol | | 12 | | -7930 | | -661 |
| Eicosanol | | 20 | | -13130 | | -656 |
We must ask ourselves whether this is a really meaningful conclusion, however. After all, these combustion values are in units of energy per mole of alcohol, but a mole of a heavier alcohol produces more products than a mole of a lighter alcohol. Because a reaction enthalpy can be thought of as stoichiometric difference in the energy released when breaking every bond of reactant molecules and the energy consumed when forming every bond of the product molecules, it shouldn't really be surprising that these values become larger for larger molecules. From a practical fuel standpoint, larger molecules have a smaller number of moles per unit volume for an identical concentration, so while burning 1 mole of butanol may release more energy than burning 1 mole of methanol, 1 gram of pure methanol (MW = 32 g/mol) has more moles of fuel than 1 gram of pure butanol (MW = 74 g/mol)... so the effect cancels out. We could easily express those combustion values as heat per gram rather than heat per mole to see the same basis effect as normalizing as "per carbon atom". but per carbon atom is what I'm going to do since it's easier.
A balanced equation for the combustion of a generic linear alcohol is:
C
nH
2n+1OH + (1.5n)O
2 
(n)CO
2 + (n+1)H
2O
Since the same two products are produced regardless of the reactant alcohol, what happens if we express the combustion enthalpy per mole of water produced, rather than per mole of alcohol consumed? Essentially, we do this by dividing by n, the number of carbons in the alcohol. (Actually we would divide by n+1, but for large molecules it effectively won't matter.) These values are also provided in the table above and are plotted below.

(Note, the figure actually shows enthalpies divided by n+1, not n. Sorry for the sloppiness.)
You can immediately see that the plot of combustion energy per mole of alcohol versus the # of carbons in the alcohol is very linear. Extrapolated to an infinitely long alcohol, the combustion enthalpy would be infinitely large! This really signifies that expressing the combustion enthalpy in this way doesn't tell you much about combustion chemistry. However, when we express the values as per carbon) produced, you can see that, with the exception of the first few alcohols, the combustion enthalpy is practically constant, no matter how many carbons are in the alcohol. It is also equal to the slope of the plot of combustion enthalpy as a function of n. In other words, the amount of energy produced per mole of water/per carbon is practically always the same, especially as n

large.
If we think about the combustion processes in terms of heats of formation, we can understand why this is the case. Combusting an alcohol of n carbon atoms requires breaking n-1 C-C single bonds, 1 C-O bond, 2n+1 C-H bonds, and 1 O-H bond. As n becomes large, almost all the bond breaking is dominated by C-C and C-H bond types, and, such that, when expressed per carbon atom, you are breaking on average one C-C bond and C-H bond. Assuming that the C-C and C-H bonds always have almost the same amount of energy no matter what the molecular structure is, you should start to see why, when normalized for the amount of carbons in the alcohol chain, the combustion enthalpy never changes. (For small alcohols, the C-O and O-H bond breakings become important in the calculation, which is why methanol and ethanol are slightly different... but not much!). Note that the combustion energy for octane, a pure hydrocarbon, is -5430 kJ/mol... which when divided by the number of carbons gives a value of -678.75 kJ/mol (of carbon). Not so different from the alcohol values. When expressed this way, all molecules that are made of primarily C-C and C-H single bonds will give around the same value, reflecting that C-C and C-H single bond energies are within an approximation almost always the same energy.
Using this information, I imagine you should be able to anticipate that your second prediction is wrong. You predict that more highly branched alcohols have lower combustion enthalpies than more linear alcohols. In most cases this is not borne out by the data. Below are tables of data for the C4 and C5 branched alcohols (and ethers, for fun), presented identically as above.
Combustion data for C-4 alcohols and ethers| Alcohol | | Identity | | ΔHc | | ΔHc per carbon atom |
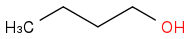 | | Alcohol | | -2670 | | -668 |
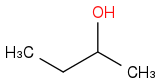 | | Alcohol | | -2661 | | -665 |
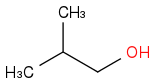 | | Alcohol | | -2668 | | -667 |
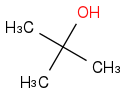 | | Alcohol | | -2644 | | -665 |
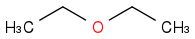 | | Ether | | -2732 | | -683 |
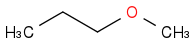 | | Ether | | -2737 | | -684 |
Combustion data for C-5 alcohols and ethers| Alcohol | | Identity | | ΔHc | | ΔHc per carbon |
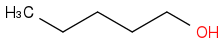 | | Alcohol | | -3327 | | -665 |
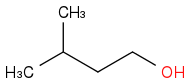 | | Alcohol | | -3326 | | -665 |
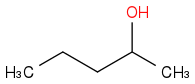 | | Alcohol | | -3315 | | -663 |
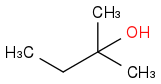 | | Alcohol | | -3303 | | -661 |
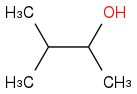 | | Alcohol | | -3315 | | -663 |
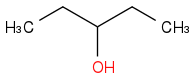 | | Alcohol | | -3312 | | -662 |
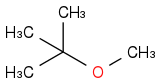 | | Ether | | -3369 | | -674 |
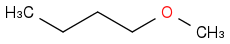 | | Ether | | -3392 | | -678 |
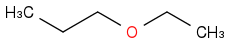 | | Ether | | -3378 | | -676 |
Here we see several things.
First, branching doesn't make much of a difference, because branching doesn't change the relative number of C-C and C-H bonds that are being broken (in a heat-of-formation consideration). This also supports the notion that in hydrocarbons and similar molecules, all C-C and C-H bonds have the same bond enthalpies, at least to a first approximation.
Second, branched C-5 and C-4 alcohols are all virtually the same, when expressed as on a "per carbon" basis.
Third, the values of the ethers are slightly different than the values for the alcohols, reflecting the fact that an extra C-O bond is being broken instead of a C-H bond. Note that the difference between the alcohols and ethers is smaller for the C-5 molecules than the C-4 molecules. This again has to do with the fact that as the molecule grows, the C-C and C-H bonds will swamp out the average combustion enthalpy per carbon.
Just to be clear, in molecules that feature other types of bonds (double bonds, say) or unusual geometries (highly strained molecules like cubane), the combustion enthalpy will change substantially, reflecting these structural modifications. But all of this data shows that for unstrained molecules that are primarily saturated hydrocarbon in nature, it doesn't particularly matter what the structure is when it comes to properly normalized combustion enthalpy - at least once you get larger than a few carbons. Of course, from a practical standpoint, there are other considerations (density, phase, and so forth) that impact utility of a substance as a fuel.
*Except when it does.
