Dear All,
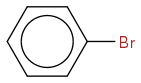
I would like some help with explaining the coupling in the spectrum of bromobenzene.
So from what I can see there should be three peaks corresponding to the three different proton environments.
Proton A (ortho to bromine) would couple to proton B (meta to Br) to produce a doublet and would then couple to proton C (Para to Br) via W coupling to produce a doublet of doublets. However looking at the spectrum there is a doublet but it has a complicated splitting pattern and I still can't explain its multiplicity even when para coupling is considered. The peaks for proton B and C are even more complicated.
I've provided a link to the spectrum (It's on page 3 of the doc)
Could anyone explain how the spectrum gets it's appearance?
Thanks
 http://www.unm.edu/~orgchem/304L%20pages/08%20Lab%201e%20Aromatics.pdf
http://www.unm.edu/~orgchem/304L%20pages/08%20Lab%201e%20Aromatics.pdf