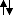So this would work, however it would not be the route I would take. It seems like it would take awhile, seeing how we would have to wait for the Ots to leave and then something to deprotonate propanol.
I would suggest adding a better leaving group to propene (anti-markov), and then adding a strong enough base to deprotonate alcohols. Think Williamson ether synthesis.