I am debugging a reaction at plant scale where there's an acid catalysed (H2SO4 95%) aldol condensation of
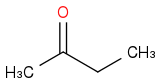
and
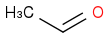
.
Typically the major product is (Product a)
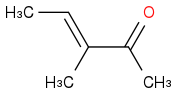
. Greater than 99.5% typically.
It so happens that something has changed that has now caused increasing amounts of ( Product c)
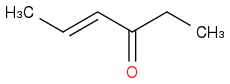
to start appearing. The amounts are still small, about 1 to 1.5% but that's still a cause of worry.
Any tips in general what are the sort of parameter deviations that one might expect to boost the selectivity of ( c )over ( a )? If Reaction temperature has wandered higher than the norm would you expect that to increase (Product c) levels?
Another point I'm suspecting is the catalyst acid, H2SO4. A supplier was recently changed and our sourcing analysis is a bit lacking. Could there be some impurity in the H2SO4 that might cause this?
Just brainstorming for ideas at the moment! Any tips?