CY231 are you trying to make maleimides?
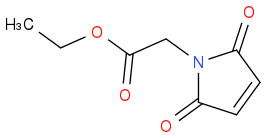
or you do you want to stop halfway?
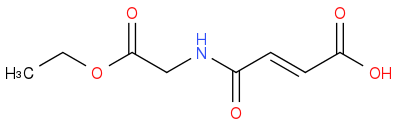
As for generating the amine free base, you can do this in situ with tertiary amine bases (with anhydrides that react only slowly with water you can even use aqueous bicarbonate with THF or 1,4-dioxane). Alternatively you can isolate the free base before coupling by extracting it from aqueous bicarbonate.
It seems to me that amide coupling reagents are not the best choice for coupling a diacid, especially if you want to stop half way and isolate the unsaturated acid product. In an amide coupling strategy, this would require the use of a monoacid followed by an additional hydrolysis step (which must be orthogonal to the ethyl ester at the other terminus); using an cyclic acid anhydride is more direct. The conditions required are likely similar to those employed for amino acid N-protection as carbamates (i.e. THF, water, bicarb) and should not cause racemisation, but I have not checked this. Racemisation normally occurs alpha to the carboxylic acid coupling partner via azlactone formation, which in this case is irrelevant (since the acid coupling partner is not the amino acid).