I'm studying about this and having problem with this exercise:
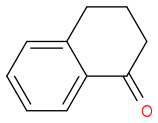
--(a)--> A --(b)--> B
a.
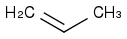
, HCl, H2O
b. 1. LDA 2. MeCl
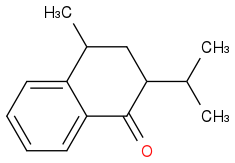
I suppose the possible structures for A and B are accordingly
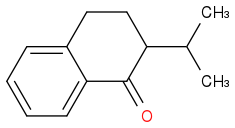
The first step: I think firstly an enol is formed and then attack the CH3-(CH+)-CH3. The friedel crafts reaction here I think is quite hard, because of the existence of C=O group.
The second: I think the iPr group there is quite big and so is LDA so the H(alpha) to the carbonyl group might be hard to be picked out, and I choose the "H" on the carbon next to the benzene.
I wonder if my guess is correct or not. Please give me some comments.