Thanks for your reply.
My first thought was like this:
1. Deprotinate with NaH

![C#[C-]](https://www.chemicalforums.com/SMILES/fdd25895122c8cdee3e1.png)
2. Add 2 Carbons by nucleophilic substitution
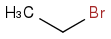

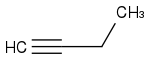
3. Deprotinate with NaH again

![[C-]#C-C-C](https://www.chemicalforums.com/SMILES/37159010ea337e1d6c7d.png)
4. Add 2 Carbons by nucleophilic substitution
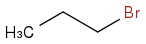

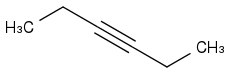
And hydrohalogenaiton with 2 equivalents of HBr would equal products of 3,3-Dibromohexane and 4,4-Dibromohexane
I just cannot figure out how to form a reaction with 3,3-Dibromohexane as a major product.
Second attempt, thoughts were like this-
Twice Halogenation of Ethyne followed by twice Elimination will not work because I can't think of any useful reactions with Dibromoethyne
Halogenation of Ethyne followed by Hydrohalogenation of 1,1-Dibromoethene will produce 1,1,2-Tribromoethane. Then twice elimination will produce Bromoethyne. Again this product does not seem useful to me because even after deprotinating, adding four carbons, having a bromine on the terminal end does not do me any more good than not having it there.
If I knew of some way to assemble carbon chains with alkenes and could produce 3-bromo-3-hexene, then a bromine could easily be added to the third carbon through hydrohalogenation.
So these are my thoughts so far.
Thanks again for reading and helping.