Shall a human determine that? It works generally without a method.
Or shall software give the answer? Then the problem gets seriously difficult. It resembles graph matching, which is known to be time-consuming.
"ANY carbon atom in my second structure that is attached to the same atoms": I guess this doesn't suffice. Checking if the atoms bonded with C are of same
nature doesn't suffice. They must have the same "identity", I mean, have bonds to atoms that themselves have the same "identity", to describe the same molecule rather than an isomer.
For instance:
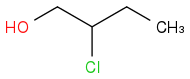
and
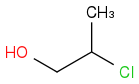
Every carbon (and every atom) of left compound has a homologue in the right compound with neighbours of the same kind, that is:
carbon #2 left corresponds to carbon #3 right and #3 left corresponds to #2 right,
but the compounds are isomers, not identical. What distinguishes #2 left from #3 right is that it bonds with a carbon bearing an OH.
Other example:
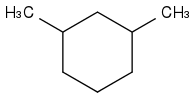
and
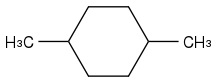
here a software can't get help from OH and Cl, and the first neighbours of -CH(CH
3)- are all -CH
2-, on the left as on the right molecule, so comparing the first neighbours doesn't suffice - nor neighbours up to some fixed distance, with a more twisted example.
In maths and software, the problem is made even more difficult by taking "atoms" of identical nature
https://en.wikipedia.org/wiki/Matching_(graph_theory)
but molecules can be huge, and then matching is a hard task. Naive methods have a complexity like N!, and complexity like N
2Log(N) demands non-trivial algorithms.