Hi everyone, I am a newbie here.
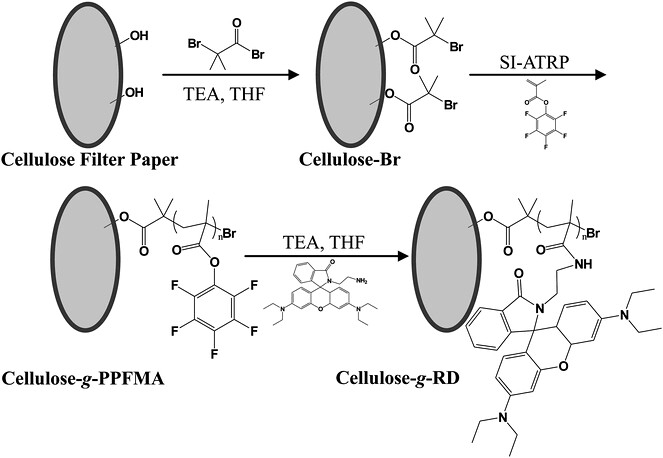
I am considering to perform atom transfer radical polymerization (ATRP) on a modified nylon surface and got issue with the very first step which involves the attachment of the initiator bromo isobutyryl bromate (BiBB) to the substrate’s OH groups. The first reaction in the figure below which I found online is pretty similar to the one I am doing. But instead of using THF, I use acetone.
This is HPLC grade solvent 99.7% pure which contains less than 0.3% water. I tried to make it further anhydrous using MgSO4. After filtration, the solvent is let stay over molecular sieve 3A overnight before use. However, upon the addition of equal molar of triethylamine (Et3N) and bromo isobutyrate bromide (Bibb), there is a significant amount of white precipitate. I think it is triethylamine bromide salt formed due to the reaction of BiBB with whatever residues left in the solvent. I suspect that there is still water left in the already dried acetone.
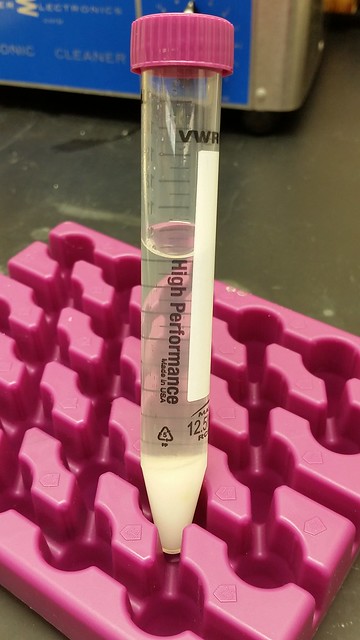
I repeated the process of drying acetone and got the same results. This actually surprised me. After drying with MgSO4 and molecular sieve, I expect there must be very very little water left. But the amount of triethylamine salt formed is quite significant. I really do not know what is going on.
How do I make sure that the acetone I use is completely free of water? Do I have to perform this step in an inert atmosphere? We do not have a glove box in the lab so maybe this will be tricky.
Another question is the role of triethylamine. I am aware that it is used as a base to neutralize the proton released in the form of HBr from the reaction of BiBB. However, I wonder what happen if triethylamine is not used? The acidic condition resulted from HBr releasing is detrimental to the proceeding of further reactions???
I really appreciate if somebody can point me in the right direction.
Thank you very much!