Borek, thank you sincerely for your response!

Yes, this is for a variety of hydroponic applications where there is no soil/soilless medium being utilized. If you are familiar with hydroponics I grow leafy vegetables in a raft type of setup, and larger plants I tend to do in an aeroponic system, if you aren't familiar and would like to know or see a little more of what I am doing, I would love to show you if interested. I am just a small hobbyist, so nothing too exciting. It's really great being able to run a high-intensity discharge light indoors, use that ambient heat to warm your house in the winter, and get fresh vegetables in the process.

I do have a ph meter as it is fairly essential when doing hydroponics to prevent a lockout of nutrients due to ph level.
Again, I must apologize for my ignorance, I am going to have to look more into this
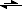
, is that the left equilibrium symbol? I think I need to pick up a book on chemistry, obviously, the
Idiot's Guide to Chemistry sounds like it would be suited for my knowledge level. Any recommendations?
Thanks for your time!